被“美女和科技”光环加持,估值高达90亿美元的Theranos昨日却遇到了问题,《华尔街日报》曝光其存在弄虚作假,据已经离职的前雇员透漏,通过几点血就能够检测多达240种指标(比如高胆固醇和癌症)的检测设备Edison其实只能检测15种,并且检测结果的可靠性和精确性也存在大大的问题,而更多的指标其实还是通过市场上已经成熟的设备检测获得的。同时,一位跟Theranos有关的律师David Boies表示,Theranos并未将其宣传的所有的血液检测在Edison设备上进行测试,而只是行进在这个目标的路途上。
Theranos创办于2003年,此前相关的报道指出,Theranos之所以能在过去十几年中一直对其技术保密,很大一部分原因是他们处于监管的灰色地带。其他诊断实验室(包括Quest和Laboratory Corporation of America)都利用从制造商处(比如西门子和罗氏诊断)购买的设备完成血液检测。在这些设备进入市场销售之前,制造商必须通过FDA的审批。如此一来,审批流程就使得检测操作技术变得更加透明。但是,Theranos生产自己需要使用的检测设备。只要他们不销售这些设备,也不将其搬离实验室,那么FDA就没必要对其进行审批。
此外,Theranos的豪华董事会也被认为是推动其业务快速扩张的重要推手,董事会成员包括前任参议院共和党多数派领袖比尔·弗里斯特,前任国务卿亨利·基辛格,前任民主党参议院、三军委员会主席山姆·纳姆,前任国防部长威廉·佩里,前任富国银行CEO兼主席理查德·科瓦西维奇等,所有这些人都接受了公司的股票期权和其他形式的酬金。91岁的基辛格曾表示:“Elizabeth Holmes(Theranos的美女CEO)有一种优雅的品质,看起来好像十九岁一般。她好像青春永驻,不会衰老。不过,她的美丽还是比不上智慧。”
针对《华尔街日报》的报道,Theranos专门在其官网发布了官方回应,表示,目前公司业务是得到大量实际客户使用证明的,并曾发表过超过1000页的相关文章证明其业务开展的有效性和可靠性,但不幸的是,《华尔街日报》断章取义,以心怀不满的前员工和行业人员对公司进行了诋毁。
并表示,任何带来变革的新技术,都会引来质疑和原有业务格局的挑战,当然在回应中我们也可以看到Theranos的相关技术和专利并未得到FDA的充分认可,目前FDA其实对其还处于观察阶段。
以下为回应全文:
Statement from Theranos
PALO ALTO, CA (October 15, 2015) – Today’s Wall Street Journal story about Theranos is factually and scientifically erroneous and grounded in baseless assertions by inexperienced and disgruntled former employees and industry incumbents. Theranos presented the facts to this reporter to prove the accuracy and reliability of its tests and to directly refute these false allegations, including more than 1,000 pages of statements and documents. Disappointingly, the Journal chose to publish this article without even mentioning the facts Theranos shared that disproved the many falsehoods in the article.
Theranos’ products and services have proven accurate and reliable for tens of thousands of satisfied customers through millions of tests and experiences and in ongoing review by our various regulators. Our focus remains on ensuring high quality, real-time, actionable information to improve diagnosis and treatment decisions. When you create innovative technology, scrutiny is to be expected. We have always welcomed that scrutiny – opening up to regulators like no lab before and voluntarily submitting all our tests for FDA review, the gold standard for quality. We received our first FDA clearance this summer based on the very proprietary systems the story is asserting don’t work, and have submitted almost 130 pre-submissions to FDA for tests run on those proprietary systems.
Theranos is working to reinvent the lab experience by providing high quality tests faster, cheaper, and more conveniently, requiring less blood, and causing less patient discomfort than ever before. We lead the industry in transparency and quality, have advocated for FDA regulation of lab tests, for the reduction of Medicare and Medicaid rates, and for transparency in pricing. We’ve partnered with health care leaders, including the Cleveland Clinic, Capital BlueCross and AmeriHealth Caritas. We also have advocated for direct access to lab testing, which will drive price transparency and lower the cost of testing in response to consumer demand; this issue is at the heart of the current movement towards individual engagement and preventive health care.
The sources relied on in the article today were never in a position to understand Theranos’ technology and know nothing about the processes currently employed by the company. We are disappointed that, in an effort to make its story more dramatic, this reporter relied only on the views of four “anonymous” disgruntled former employees, competitors and their allies, instead of reaching out to many of the scientific, health care and business leaders who have actually seen, tested, used and examined our breakthrough technologies. The Journal even declined an opportunity to experience the technology themselves by turning down our offer to send proprietary Theranos devices to their offices so they could have a demonstration of tests conducted themselves, and compare the results to those of other testing providers.
Stories like this come along when you threaten to change things, seeded by entrenched interests that will do anything to prevent change, but in the end nothing will deter us from making our tests the best and of the highest integrity for the people we serve, and continuing to fight for transformative change in health care.
医谷链
来源:医谷网
为你推荐
 资讯
资讯 天津市互联网诊疗监管实施办法(试行)
医疗机构应当主动与市级监管平台对接,及时上传、更新《医疗机构执业许可证》等相关执业信息,主动接受监督。医疗机构取得《医疗机构执业许可证》后或《医疗机构执业许可证》变...
2026-02-06 08:59
 资讯
资讯 八部委发布《中药工业高质量发展实施方案(2026—2030年)》
培育60个高标准中药原料生产基地。协同体系更加健全,中药材种植加工、中药研发生产、流通服务等上下游各环节协同更加紧密,建设5个中药工业守正创新中心,推动一批中药创新药获...
2026-02-05 21:21
 资讯
资讯 国家医保局今年将重点对精神类定点医疗机构开展专项飞检
各省级医保部门要组织本辖区内所有精神类定点医疗机构从即日起全面开展自查自纠,重点聚焦但不限于诱导住院、虚假住院、虚构病情、虚构诊疗、伪造文书、违规收费等违法违规使用...
2026-02-05 17:13
 资讯
资讯 国际SOS荣膺“2026年度全球杰出雇主”
今日,国际SOS宣布,公司连续第八年荣获杰出雇主调研机构(Top Employers Institute)授予的杰出雇主认证。
2026-02-05 14:42
 资讯
资讯 默克高管周虹离任,诺和诺德官宣在即,医药行业再迎关键人事变动
默克医药健康全球执行副总裁、中国及国际市场负责人周虹正式离任,其将加盟丹麦制药巨头诺和诺德,接任产品与组合战略执行副总裁一职
2026-02-05 11:58
 资讯
资讯 104亿元!2026年小核酸领域首笔出海BD诞生
圣因生物与罗氏集团旗下子公司基因泰克达成全球研发合作与许可协议,双方将基于圣因生物专有的RNAi药物研发平台,共同推进一款RNAi疗法的开发。
2026-02-05 11:50
 资讯
资讯 合成生物企业桦冠生物宣布完成数亿元C轮融资
本轮融资由软银欣创、顺禧基金、常州启航合成生物创投基金、国投创益、长江资本等多家知名机构联合投资,光源资本担任财务顾问,所融资金将重点投向医药与大健康领域新品研发、...
2026-02-04 11:50
 资讯
资讯 AI制药独角兽深度智耀完成6000万美元新一轮融资,加速全栈式研发解决方案落地
本轮融资吸引了信宸资本、金镒资本、凯泰资本等新投资方入局,老股东鼎晖百孚、新鼎资本持续追加投资
2026-02-03 20:03
 资讯
资讯 济川药业联合康方生物,共拓心血管创新药商业化新局
伊喜宁®(伊努西单抗注射液)是康方生物自主开发创新的PCSK9单克隆抗体新药,于2024年9月获批上市,用于治疗原发性高胆固醇血症和混合型高脂血症,包括杂合子家族性高胆固醇血...
2026-02-03 19:34
拜耳诺倍戈®第三项适应症在中国获批,用于治疗转移性激素敏感性前列腺癌(mHSPC)
诺倍戈®此前已先后获批用于治疗有高危转移风险的非转移性去势抵抗性前列腺癌(NM-CRPC)成年患者,和联合多西他赛治疗转移性激素敏感性前列腺癌的(mHSPC)成年患者。
2026-02-03 18:42
 资讯
资讯 又一款老花眼滴眼液获批,市场有待检验
近日,Tenpoint Therapeutics宣布,美国FDA已批准Yuvezzi(carbachol和brimonidine tartrate滴眼液,2 75% 0 1%),用于治疗成人老花眼。
2026-02-02 14:06
 资讯
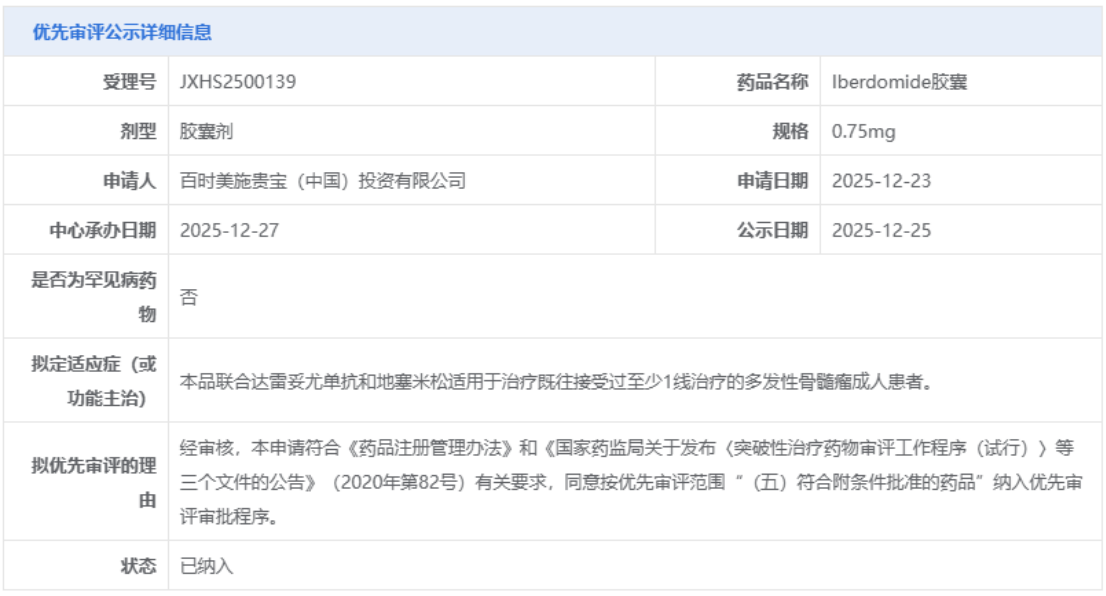
资讯 深度缓解数据惊艳:基于诺奖机制的 CELMoD药物如何重塑 MM 治疗逻辑?
近期,国家药品监督管理局药品审评中心(CDE)将CELMoD药物 iberdomide (以下简称:IBER)纳入优先审评名单并启动优先审评程序,拟批准
2026-02-02 13:22
 资讯
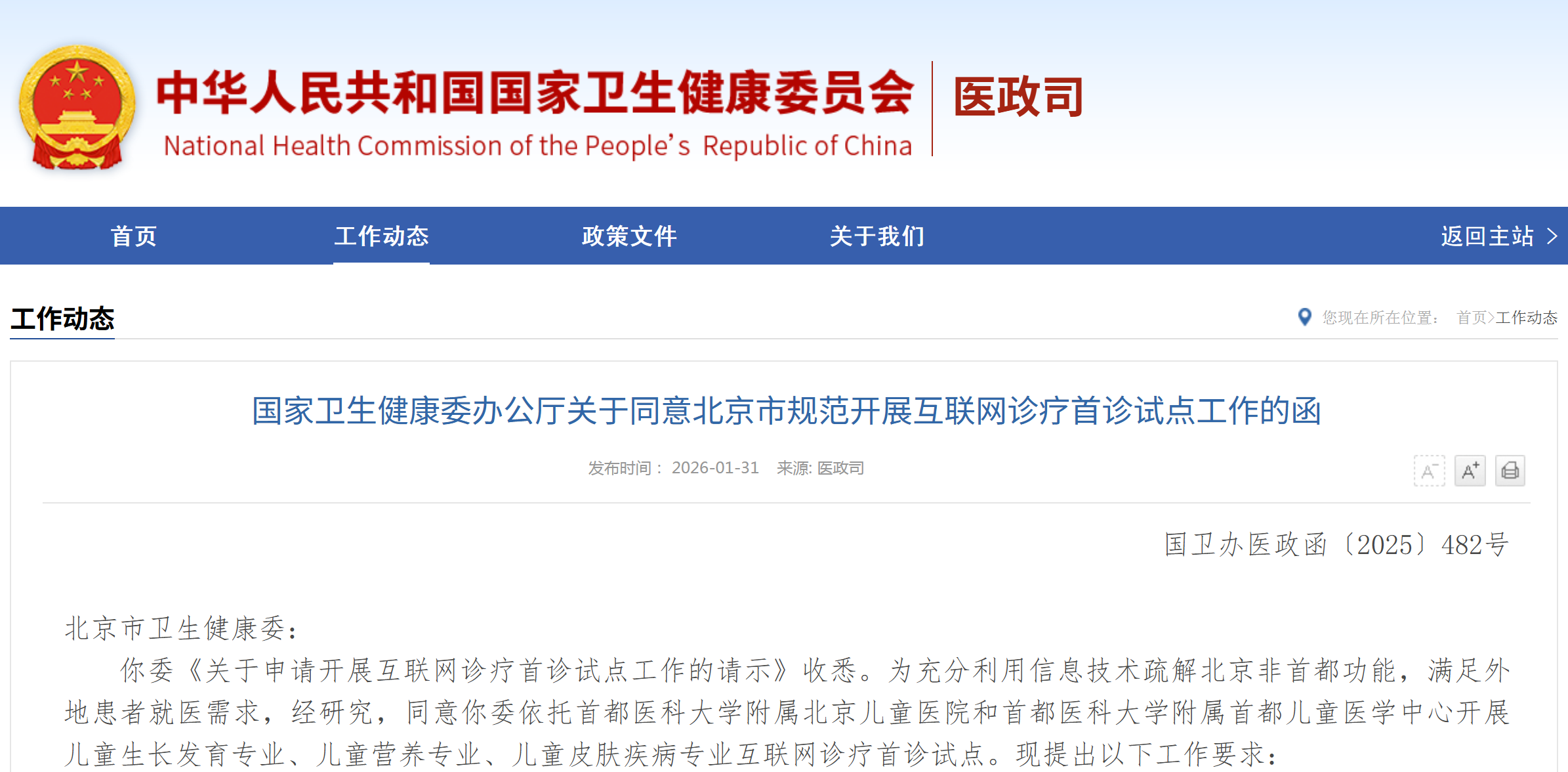
资讯 儿童生长发育专业、儿童营养专业、儿童皮肤疾病专业纳入北京互联网诊疗首诊试点
经研究,同意你委依托首都医科大学附属北京儿童医院和首都医科大学附属首都儿童医学中心开展儿童生长发育专业、儿童营养专业、儿童皮肤疾病专业互联网诊疗首诊试点。
2026-01-31 23:34